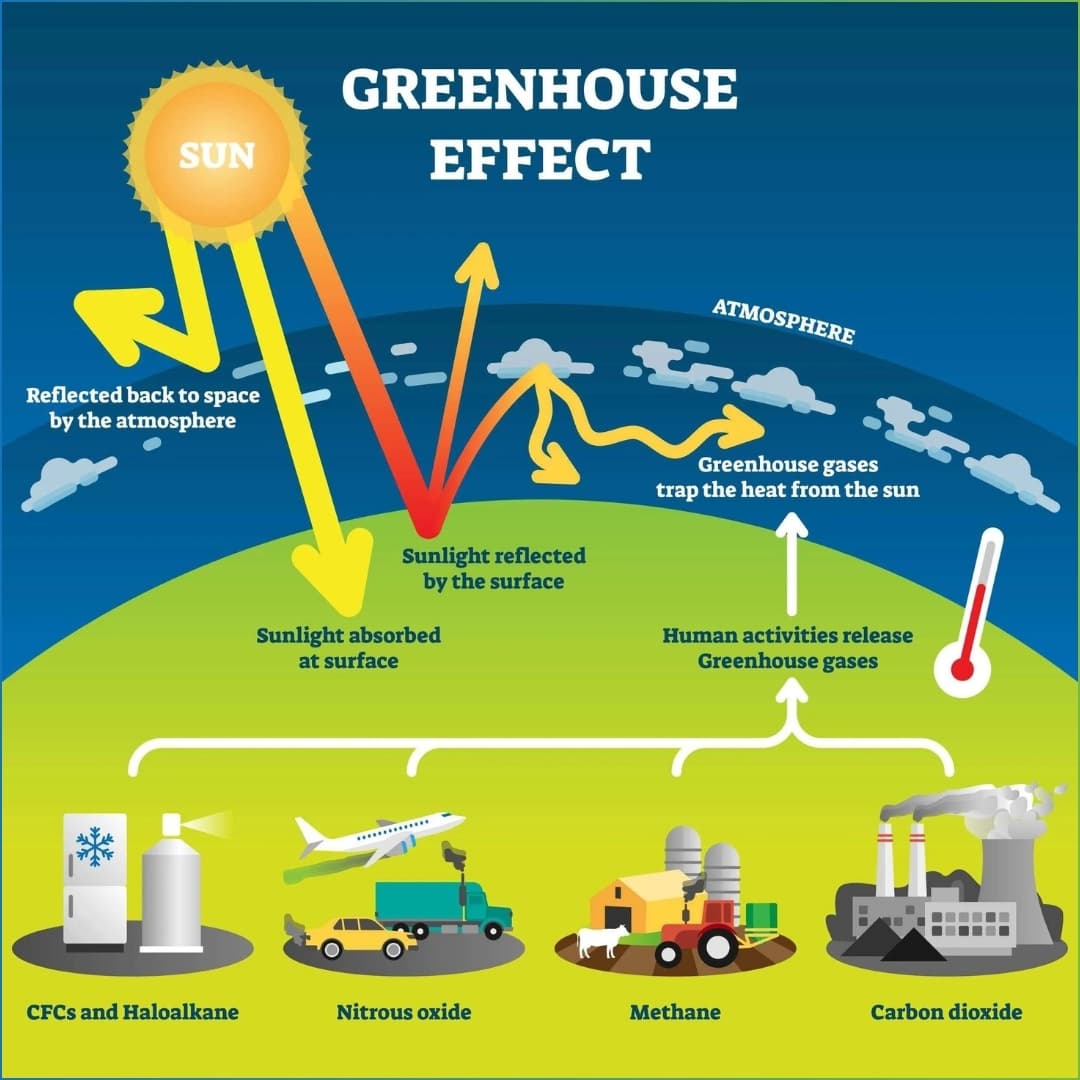
Greenhouse Gases are gases in the atmosphere that help Mother Earth trap heat from the sun’s rays. The atmosphere mainly consists of these six Greenhouse gases: Carbon Dioxide, Nitrous Oxide, Ozone, Methane, Water Vapour and Chlorofluorocarbons. These gases not only have positive but also negative impacts on our planet.
Carbon dioxide is made up of two atoms of oxygen and an atom of carbon. Its chemical formula is represented as CO2. It is released through volcanoes, forest fires, and decaying of dead animals and plants. It is emitted from the burning of fossil fuels like coal, oil and petroleum. Factories and vehicles emit this greenhouse gas. Deforestation is yet another cause that leads to excessive carbon dioxide in the atmosphere.
The next gas, Nitrous Oxide (N2O) also called ‘the laughing gas’ is released from power plants and fertilizers. N2O is found in nature in oceans and soil. This greenhouse gas is a part of the nitrogen cycle.
The most powerful greenhouse gas of all, Methane is also known as CH4. It is a natural greenhouse gas that is released through cattle and wetlands. Mining coal and growing rice are manmade causes of adding Methane to our environment.
Ozone, O3 blocks the sun’s harmful ultraviolet radiation to reach us. It is the layer of the atmosphere where the planes fly. It is released from burning gas from cars and factories.
Chlorofluorocarbons are the only manmade greenhouse gas. There is no natural source for this greenhouse gas, CFCs. It damages the Ozone layer.

Excess CFCs are released by manmade devices like refrigerators, fire extinguishers, air conditioners, and aerosol sprays, etc.
Water Vapour is water in a gaseous form. This gas condenses into liquid form and rains back on Earth. H2O, the water which we drink is a part of this natural cycle. Water Vapour blocks heat from escaping the atmosphere making it more warmer.
We need to reduce emitting greenhouse gases to help our planet become a better place to live. ‘Going Green’ is one of the best ways to reduce ‘Greenhouse Gas Emissions’.









